Surfactants play a crucial role in a variety of applications, from cleaning agents to personal care products and industrial processes. These versatile compounds have unique properties that make them essential in countless products we use daily. In this article, we'll explore what sets surfactants apart, examine the different types, and understand how their structure influences performance.
Surfactants, short for surface-active agents, are compounds that reduce the surface tension between two substances, typically a liquid and a gas or liquid and solid. By doing so, they enable the mixture to spread more easily or penetrate surfaces. The name "surfactant" derives from the combination of "surface" and "active," indicating their ability to interact with and alter the properties of surfaces.
These compounds are amphiphilic, meaning they possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) properties. This dual nature allows surfactants to interact with various substances, making them incredibly versatile.
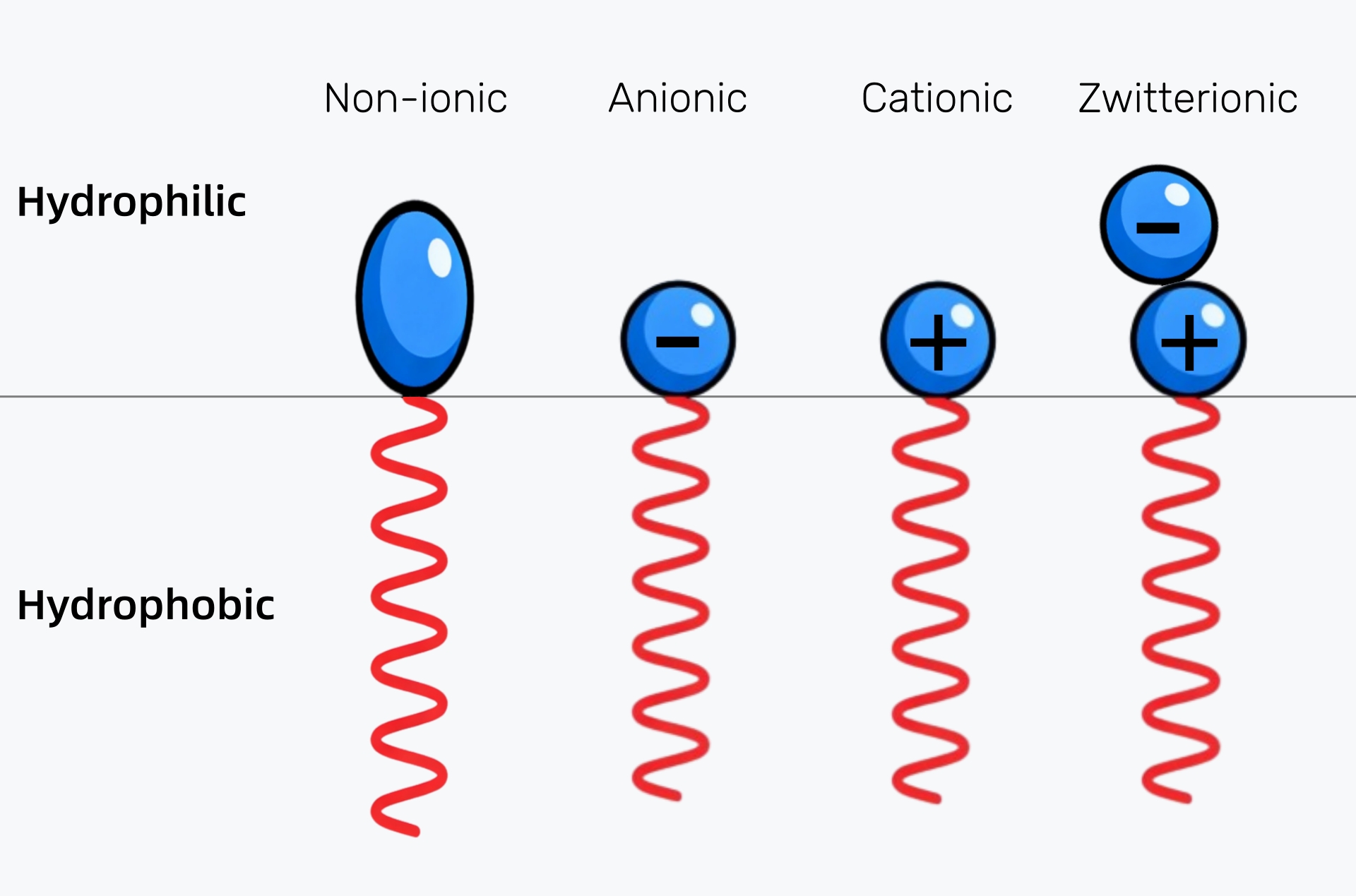
Surfactants can be categorized into four main types based on their charge: anionic, cationic, nonionic, and amphoteric. Each type has distinct characteristics and is used in different industries for specific functions.
Anionic surfactants are the most commonly used and have a negative charge on their hydrophilic end. They are highly effective in cleaning, as their negative charge allows them to interact with and lift dirt, grease, and oils. Common examples include sodium lauryl sulfate (SLS) and sodium dodecylbenzenesulfonate. These are typically found in household detergents, shampoos, and soaps.
Cationic surfactants, which have a positive charge on their hydrophilic end, are primarily used for conditioning and antimicrobial purposes. They are commonly found in fabric softeners, hair conditioners, and disinfectants due to their ability to neutralize static and kill bacteria.
Nonionic surfactants do not carry a charge, making them more stable in a wider range of pH conditions and less irritating to the skin. They are frequently used in cosmetics, cleaning products, and emulsifiers, such as in lotions, creams, and food products. Examples include polysorbate 80 and ethoxylated alcohols.
Zwitterionic surfactants can carry either a positive or negative charge, depending on the pH of the solution. This versatility makes them ideal for personal care products, where mildness is crucial. Common examples include cocamidopropyl betaine, often used in shampoos and facial cleansers.
While surfactants are key ingredients in many cleaning products, not all surfactants are detergents, and the two terms are often used interchangeably but are not quite the same.
Surfactants are the active compounds that work to lower surface tension, allowing the cleaning or emulsifying process to occur. They may or may not be formulated into a final cleaning product. Surfactants can function in a variety of roles beyond cleaning, such as foaming agents, emulsifiers, and stabilizers.
Detergents, on the other hand, are specifically formulated cleaning products that contain surfactants as one of the primary ingredients. Detergents typically include a blend of surfactants, builders (to enhance cleaning performance), and other additives that improve their efficacy. In essence, all detergents are surfactants, but not all surfactants are detergents.
The structure of a surfactant plays a significant role in determining its effectiveness in various applications. The amphiphilic nature of surfactants, with one hydrophilic (water-attracting) and one hydrophobic (water-repelling) part, is key to their ability to lower surface tension.
The hydrophobic part of the molecule binds to oils and grease, while the hydrophilic part remains in the water. This enables surfactants to break down and emulsify oils, fats, and dirt, making them easier to remove during cleaning. The length of the hydrophobic tail and the size of the hydrophilic head affect the surfactant's solubility and foaming properties.
The size of the hydrophilic group and the strength of the hydrophobic interaction also impact the surfactant's foaming ability. For example, surfactants with longer hydrophobic tails tend to create more stable foam, which is desirable in products like shampoos and soaps. However, excessive foam can sometimes reduce cleaning efficiency, so product formulation must balance foam generation with cleaning power.
The structure of surfactants can also make them more or less effective in different temperature and pH environments. Some surfactants are sensitive to heat or acidic conditions, causing them to lose their effectiveness. Nonionic surfactants, for example, are generally more stable across a wider range of temperatures and pH levels, making them ideal for diverse formulations.
Surfactants are diverse and essential ingredients in many products, ranging from cleaning agents to personal care formulations. Understanding the differences between surfactants and detergents, as well as how their structure influences their performance, is key to selecting the right surfactant for any given application. Whether you're formulating a new cleaning product or choosing the best personal care item, knowing how surfactants work can help you make informed decisions about product efficacy and safety.
By mastering the science behind surfactants, we gain better insights into their functions, potential applications, and environmental impact. This knowledge helps ensure that these versatile compounds are used effectively in industries ranging from cleaning to cosmetics, agriculture, and beyond.